- AGROMYZIDAE
- AGROMYZINAE
- AGROMYZA
- Agromyza abdita
- Agromyza abiens
- Agromyza albipennis
- Agromyza albitarsis
- Agromyza alnibetulae
- Agromyza alnivora
- Agromyza alunulata
- Agromyza ambigua
- Agromyza anthracina
- Agromyza audcenti
- Agromyza bicaudata
- Agromyza bicophaga
- Agromyza bromi
- Agromyza cinerascens
- Agromyza conjuncta
- Agromyza demeijerei
- Agromyza dipsaci
- Agromyza erythrocephala
- Agromyza felleri
- Agromyza ferruginosa
- Agromyza filipendulae
- Agromyza flaviceps
- Agromyza flavipennis
- Agromyza frontella
- Agromyza graminicola
- Agromyza hendeli
- Agromyza idaeiana
- Agromyza igniceps
- Agromyza intermittens
- Agromyza johannae
- Agromyza lathyri
- Agromyza lithospermi
- Agromyza lucida
- Agromyza luteitarsis
- Agromyza macedonica
- Agromyza marionae
- Agromyza mobilis
- Agromyza myosotidis
- Agromyza nana
- Agromyza nigrella
- Agromyza nigrescens
- Agromyza nigripes
- Agromyza nigrociliata
- Agromyza phragmitidis
- Agromyza polygoni
- Agromyza prespana
- Agromyza pseudoreptans
- Agromyza pseudorufipes
- Agromyza pulla
- Agromyza reptans
- Agromyza rondensis
- Agromyza rubiginosa
- Agromyza rufipes
- Agromyza sulfuriceps
- Agromyza varicornis
- Agromyza viciae
- Agromyza vicifoliae
- HEXOMYZA
- MELANAGROMYZA
- Melanagromyza aenea
- Melanagromyza aeneoventris
- Melanagromyza albocilia
- Melanagromyza angeliciphaga
- Melanagromyza astragali
- Melanagromyza cunctans
- Melanagromyza dettmeri
- Melanagromyza eriolepidis
- Melanagromyza eupatorii
- Melanagromyza fabae
- Melanagromyza galegae
- Melanagromyza lappae
- Melanagromyza limata
- Melanagromyza moatesi
- Melanagromyza nartshukae
- Melanagromyza nibletti
- Melanagromyza oligophaga
- Melanagromyza pubescens
- Melanagromyza sativae
- Melanagromyza symphyti
- Melanagromyza tripolii
- OPHIOMYIA
- Ophiomyia alliariae
- Ophiomyia aquilegiana
- Ophiomyia beckeri
- Ophiomyia collini
- Ophiomyia cunctata
- Ophiomyia curvipalpis
- Ophiomyia definita
- Ophiomyia galii
- Ophiomyia gnaphalii
- Ophiomyia heracleivora
- Ophiomyia heringi
- Ophiomyia labiatarum
- Ophiomyia longilingua
- Ophiomyia maura
- Ophiomyia melandricaulis
- Ophiomyia melandryi
- Ophiomyia nasuta
- Ophiomyia ononidis
- Ophiomyia orbiculata
- Ophiomyia penicillata
- Ophiomyia pinguis
- Ophiomyia pulicaria
- Ophiomyia ranunculicaulis
- Ophiomyia rostrata
- Ophiomyia senecionina
- Ophiomyia ungarensis
- AGROMYZA
- PHYTOMYZINAE
- AMAUROMYZA
- AULAGROMYZA
- Aulagromyza anteposita
- Aulagromyza buhri
- Aulagromyza cornigera
- Aulagromyza discrepans
- Aulagromyza fulvicornis
- Aulagromyza hendeliana
- Aulagromyza heringii
- Aulagromyza lucens
- Aulagromyza luteoscutellata
- Aulagromyza orphana
- Aulagromyza populi
- Aulagromyza populicola
- Aulagromyza similis
- Aulagromyza tremulae
- Aulagromyza tridentata
- Aulagromyza trivittata
- CALYCOMYZA
- CERODONTHA
- CHROMATOMYIA
- Chromatomyia aprilina
- Chromatomyia asteris
- Chromatomyia autumnalis
- Chromatomyia blackstoniae
- Chromatomyia centaurii
- Chromatomyia farfarella
- Chromatomyia fuscula
- Chromatomyia glacialis
- Chromatomyia horticola
- Chromatomyia isicae
- Chromatomyia lonicerae
- Chromatomyia luzulae
- Chromatomyia milii
- Chromatomyia nigra
- Chromatomyia obscuriceps
- Chromatomyia opacella
- Chromatomyia periclymeni
- Chromatomyia primulae
- Chromatomyia ramosa
- Chromatomyia scabiosae
- Chromatomyia scolopendri
- Chromatomyia spinaciae
- Chromatomyia succisae
- Chromatomyia syngenesiae
- GYMNOPHYTOMYZA
- LIRIOMYZA
- Liriomyza amoena
- Liriomyza angulicornis
- Liriomyza artemisicola
- Liriomyza bryoniae
- Liriomyza cannabis
- Liriomyza centaureae
- Liriomyza cicerina
- Liriomyza congesta
- Liriomyza demeijerei
- Liriomyza equiseti
- Liriomyza erucifolii
- Liriomyza eupatoriana
- Liriomyza eupatorii
- Liriomyza europaea
- Liriomyza flaveola
- Liriomyza flavopicta
- Liriomyza hampsteadensis
- Liriomyza hieracii
- Liriomyza huidobrensis
- Liriomyza infuscata
- Liriomyza intonsa
- Liriomyza latigenis
- Liriomyza latipalpis
- Liriomyza lutea
- Liriomyza morio
- Liriomyza obliqua
- Liriomyza occipitalis
- Liriomyza orbona
- Liriomyza pascuum
- Liriomyza pedestris
- Liriomyza phryne
- Liriomyza pisivora
- Liriomyza polygalae
- Liriomyza ptarmicae
- Liriomyza puella
- Liriomyza pusilla
- Liriomyza pusio
- Liriomyza richteri
- Liriomyza scorzonerae
- Liriomyza solivaga
- Liriomyza sonchi
- Liriomyza soror
- Liriomyza strigata
- Liriomyza tanaceti
- Liriomyza taraxaci
- Liriomyza taurica
- Liriomyza tragopogonis
- Liriomyza trifolii
- Liriomyza valerianae
- Liriomyza violiphaga
- Liriomyza virgo
- Liriomyza virgula
- Liriomyza yasumatsui
- METOPOMYZA
- NAPOMYZA
- NEMORIMYZA
- PHYTOBIA
- PHYTOLIRIOMYZA
- Phytoliriomyza alpicola
- Phytoliriomyza arctica
- Phytoliriomyza dorsata
- Phytoliriomyza hilarella
- Phytoliriomyza immoderata
- Phytoliriomyza melampyga
- Phytoliriomyza nigrifrons
- Phytoliriomyza ornata
- Phytoliriomyza pectoralis
- Phytoliriomyza perpusilla
- Phytoliriomyza pteridii
- Phytoliriomyza scotica
- Phytoliriomyza venustula
- PHYTOMYZA
- Phytomyza aconiti
- Phytomyza adjuncta
- Phytomyza affinis
- Phytomyza agromyzina
- Phytomyza albiceps
- Phytomyza albipennis
- Phytomyza alpina
- Phytomyza anderi
- Phytomyza anemones
- Phytomyza angelicae
- Phytomyza angelicastri
- Phytomyza antennariae
- Phytomyza aquilegiae
- Phytomyza arnicae
- Phytomyza artemisivora
- Phytomyza astrantiae
- Phytomyza bipunctata
- Phytomyza brischkei
- Phytomyza brunnipes
- Phytomyza buhriella
- Phytomyza calthivora
- Phytomyza calthophila
- Phytomyza campanulae
- Phytomyza cecidonomia
- Phytomyza chaerophylli
- Phytomyza cineracea
- Phytomyza cinerea
- Phytomyza cirsii
- Phytomyza clematidis
- Phytomyza conii
- Phytomyza continua
- Phytomyza conyzae
- Phytomyza corvimontana
- Phytomyza crassiseta
- Phytomyza cytisi
- Phytomyza dasyops
- Phytomyza diversicornis
- Phytomyza enigmoides
- Phytomyza erigerophila
- Phytomyza eupatorii
- Phytomyza evanescens
- Phytomyza fallaciosa
- Phytomyza ferina
- Phytomyza flavicornis
- Phytomyza flavofemorata
- Phytomyza fulgens
- Phytomyza glechomae
- Phytomyza griffithsi
- Phytomyza gymnostoma
- Phytomyza heckfordi
- Phytomyza hellebori
- Phytomyza hendeli
- Phytomyza heracleana
- Phytomyza heringiana
- Phytomyza heterophylli
- Phytomyza ilicis
- Phytomyza isais
- Phytomyza kaltenbachi
- Phytomyza krygeri
- Phytomyza lappae
- Phytomyza leucanthemi
- Phytomyza marginella
- Phytomyza medicaginis
- Phytomyza melana
- Phytomyza minuscula
- Phytomyza murina
- Phytomyza myosotica
- Phytomyza nigrifemur
- Phytomyza nigripennis
- Phytomyza nigritella
- Phytomyza nigritula
- Phytomyza notata
- Phytomyza obscura
- Phytomyza obscurella
- Phytomyza origani
- Phytomyza orobanchia
- Phytomyza paraciliata
- Phytomyza pastinacae
- Phytomyza pauliloewii
- Phytomyza pedicularifolii
- Phytomyza penicilla
- Phytomyza petoei
- Phytomyza phillyreae
- Phytomyza pimpinellae
- Phytomyza plantaginis
- Phytomyza pullula
- Phytomyza ranunculella
- Phytomyza ranunculi
- Phytomyza ranunculicola
- Phytomyza ranunculivora
- Phytomyza rhabdophora
- Phytomyza rhodiolae
- Phytomyza robustella
- Phytomyza rostrata
- Phytomyza rufescens
- Phytomyza rufipes
- Phytomyza rydeni
- Phytomyza schlicki
- Phytomyza scotina
- Phytomyza sedi
- Phytomyza sedicola
- Phytomyza silai
- Phytomyza sitchensis
- Phytomyza soenderupi
- Phytomyza solidaginis
- Phytomyza sphondyliivora
- Phytomyza spoliata
- Phytomyza spondylii
- Phytomyza stolonigena
- Phytomyza tanaceti
- Phytomyza tenella
- Phytomyza tetrasticha
- Phytomyza thysselini
- Phytomyza trollii
- Phytomyza trolliivora
- Phytomyza trolliophila
- Phytomyza tussilaginis
- Phytomyza varipes
- Phytomyza virgaureae
- Phytomyza vitalbae
- Phytomyza wahlgreni
- PSEUDONAPOMYZA
- AGROMYZINAE
Family
AGROMYZIDAESubfamily
AGROMYZINAEGenus
MELANAGROMYZASpecies
Melanagromyza astragali Spencer, 1976
Mine
N/A. An internal stem-borer. Feeding within the stems. Pupariation internal.
Host Plants
Confusion Species
N/A.
Recording Grade
5
National Status
pRDB 1.
Puparium
Unknown. No puparia are present with holotype or paratypes in the MZLU or NHMUK collections, respectively. Spencer (1976) did not discuss larval/puparial details when describing the species.
Adult
Head: Frons 1.5-1.6x as wide as eye, orbits slightly projecting above eye in profile, with 2 strong ors, 2 strong ori. Orbital setulae relatively sparse, in a single row, all reclinate. Eye bare in both sexes. Gena slightly deeper in centre below eye, 0.14-0.20x as high as eye. Cheek barely forming a ring below eye. Orbits relatively narrow; lunule semi-circular, sometimes with a distinct central furrow. Ocellar triangle reaching to level of lower ors, sometimes to upper ori. Third antennal segment small, rounded with short pubescence, not longer than the basal section of the arista.
Mesonotum: 2 strong dc; acr in 8-10 rows, reducing to 4-5 at level of first dc. Prescutellar acrostichal setae, shorter than dc, frequently present (including in holotype and paratypes, not detected by Spencer); 5 out of 7 specimens examined by the author possess distinct prsc. Material examined by Owen Lonsdale (pers. comm.) also possess prsc; out of 4 specimens, 3♂ with prsc (asymmetrical in 1), 1 without prsc. Frequently, 1-6 lateral to dorsolateral scutellar setulae close to lateral scutellar setae (including in holotype, not detected by Spencer) are present.
Wing: Hyaline, ultimate section of vein M4 0.80 to about equal that of the penultimate. Costal sections (2-4) with an average ratio of 100:29:24. Length 2.1-2.2 mm in male, 2.5 mm in female.
Colour: Frons matt, deep black. Orbits slightly shining, ocellar triangle weakly shining. Lunule slightly paler than frons. Gena brownish-black, palpi black. Mesonotum and abdomen black, shining, with no coppery tinge. Calypter greyish, margin dark, black to dark brown, fringe paler, light brown. Legs with 2 (rarely 3) posterolateral setae on mid tibia. In smaller specimens, the posterolateral setae are frequently absent.
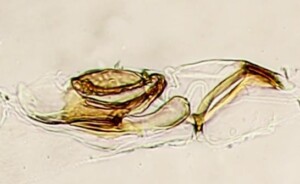
Male genitalia: Surstylus possessing a group of long setae and several spines on inner surface; basiphallus ring-like, very close to mesophallus; basal bladder of mesophallus with slight upward curvature; distiphallus with weak areas of spiculae when viewed from above; distal tubule upcurved and rather weak.
Female genitalia: Egg guide acute apically, 4.5x as long as maximum width, medial membrane with numerous sensilla centrally between inner and outer margins.
Additional information
Taken from Warrington 2024:
This species was added to the British list by Bland (2001), based on material reared from puparia in the dead stems of Astragalus glycyphyllos in South Essex. In total, Keith Bland reared three females (pers. comm.), two of which were kindly loaned to me for the purpose of this revision. Upon examination, both specimens are not conspecific with the type description of astragali by Spencer (1976); both females possess distinctly proclinate orbital setulae, which are in two rows (astragali is stated to possess entirely reclinate orbital setulae in a single row) and a pale margin to the calypter (dark in astragali).
High resolution images of the holotype (held in Biological Museum, Lund University, Sweden) were kindly sent to me; upon examination, Spencer’s original description is accurate, the only omissions being that the holotype possesses four erect orbital setulae and one slightly proclinate setula, prsc are present, with the scutellum possessing one small scutellar setula close to each lateral scutellar seta.
The orbital setulae in the dissected male specimens discussed by Warrington and Perry (2023) are reclinate, with the calypter margin being dark, brownish, agreeing with the description by Spencer. Material (2♂) in the von Tschirnhaus collection also possess reclinate orbital setulae and agree with the description by Spencer (Michael von Tschirnhaus pers. comm.). Considering the significant differences between the female material reared by Keith Bland, the type specimen of astragali and the dissected material mentioned above, it seems only prudent that the record by Keith Bland is hereby excluded, with the first formal British records being those published by Warrington and Perry.
The Bland specimens were reared from puparia possessing dark, very closely-spaced posterior spiracles, each with 14-16 bulbs. Of the known British species, only M. angeliciphaga, M. galegae and M. moatesi agree in terms of posterior spiracular detail and entirely proclinate orbital setulae. The pale calypter margin of the Bland material differs from galegae and moatesi, whilst angeliciphaga is a much larger species (even allowing for natural variation). It seems probable that the material reared by Keith relates to another possibly undescribed species. In Great Britain, only M. pubescens is similar to astragali, owing to the dark calypter margin/fringe and reclinate orbital setulae but that species is larger in size, possesses a darker calypter fringe and strikingly different male genitalia.
Distribution
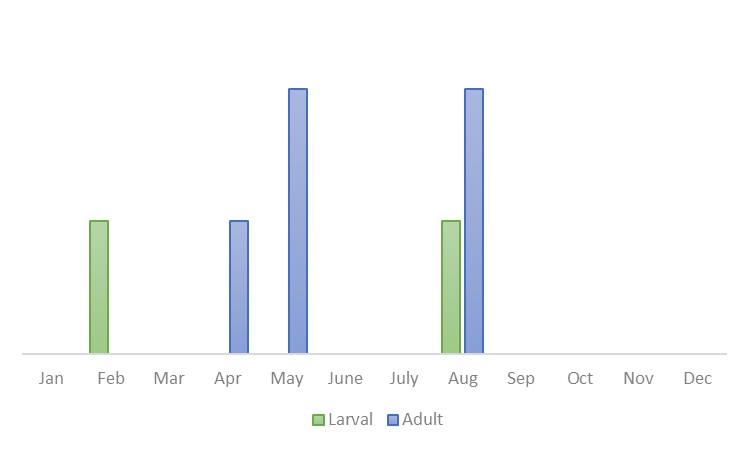
Adult
Head and squama
Genitalia