Parasitoids of Agromyzidae by Professor Sir Charles Godfray
Anyone who tries to rear agromyzids will quickly encounter their numerous parasitoid wasps. Some of these are astoundingly beautiful insects, at least when examined with a good lens or binocular microscope. These notes are aimed to be an introduction to agromyzid parasitoids, and an entry into the relevant literature. These wasps are fascinating in themselves, and there is still much to be discovered, but are also an important scientific resource. The food webs centred on leafminers and their parasitoids are some of the best understood of all plant-herbivore-natural enemy webs and have provided significant ecological insights. Please don’t discard your parasitoids!
Biology
Parasitoids are insects whose larvae develop by feeding on the body of another insect. They differ from parasites in invariably killing their hosts, and from predators in only requiring one host insect for their complete development. Nearly all agromyzid parasitoids are solitary, only one parasitoid developing per host; in other systems several to hundreds of gregarious parasitoids develop on a single host. Agromyzid parasitoids have two main types of biology:
Ectoparasitoids. A female parasitoid locates a host and stings it, leaving it paralysed, and lays an egg on its body. The egg hatches and the larva feeds externally on the host, pupating in the mine or feeding gallery. Feeding larvae or pupae can easily be seen in back-lit leafmines (Fig. 1). Because the agromyzid does not grow after attack, some of the parasitoids that develop on young hosts are very small, much smaller than the fly. Typically, males develop on small hosts and females on larger hosts.

Fig. 1. The dead larva to the right has been sucked dry by an ectoparasitoid eulophid wasp which has pupated to its left within the leaf mine. The adult parasitoid hatches in the mine and uses its mandibles to fashion an exit hole.
Endoparasitoids. The female parasitoid locates a host larva (occasionally a pupa in the leaf) and lays an egg inside its body; the agromyzid is either not paralysed or only temporally so – it recovers and continues to grow, eventually pupating inside or outside the mine. During this period the parasitoid suspends development, typically as a first instar larva. Only when the host pupates does the parasitoid resume development, consuming all available host tissue, before pupating within the hosts puparium. These parasitoids are about the same size as the fly.
If a parasitoid encounters a host that is already parasitised then it may kill or parasitise the first parasitoid. If the first parasitoid is of the same species then it is called superparasitism and if a different species facultative hyperparasitism. In other systems there are obligate hyperparasitoids: species that can only develop as parasitoids of parasitoids.
Taxonomy
In this section I describe the main parasitoid taxa attacking agromyzids, leaving out a few less common or unusual taxa.
The main division is between Ichneumonoidea and Chalcidoidea (Fig. 2). These are very different looking insects which cannot be confused (for example see wing venation and antennal structure). Most (but not all) chalcidoids attacking agromyzids are wholly or partly coloured metallic green or blue.

Fig. 2. Ichneumonoidea (left) can be distinguished from Chalcidoidea (right) through their relatively complete venation and their antennae which have more than 13 segments (typically many more). The species on the left is Chorebus cylindricus (Braconidae, Alysiinae, Dacnusini) which is a parasitoid of Melanagromyza spp. in plant stems (notice the relatively long ovipositor). On the right is a male Chrysocharis orbicularis (Eulophidae, Entedontinae) which attacks a number of leaf-mining agromyzids from different genera.
Ichneumonoidea
The Ichneumonoidea attacking agromyzids are (virtually) all in the family Braconidae and with one exception are endoparasitoids that parasitise young larva and emerge from the pupae. A major group, the vast majority of which are agromyzid parasitoids, is the Dacnusini, a tribe of the subfamily Alysiinae. These can be distinguished by the absence of a vein in the forewing (Fig. 3).

Fig. 3. Dacnusini (left) lack the cross-vein r-m found in most other Braconidae (indicated by red arrow on right). The specimen on the left is from a group of Exotela spp. (Braconidae, Alysiinae, Dacnusini), sometimes placed in the genus Anthusa, which attack agromyzids leaf-mining grasses. DNA sequence analysis suggests there are a complex of species whose morphological and biological limits are not yet fully understood. On the right is Opius pallipes (Braconidae, Opiinae), one of the commonest species of the subfamily, which has been reared and sold commercially for biological control against Liriomyza spp. in greenhouses.
There are nearly 200 Dacnusini species in the UK, largely in the genera Chorebus (Fig. 4), Dacnusa (Fig. 5) and Exotela (Fig. 3 & 6). Many are extremely host specific, and there are species groups specialised on non-leaf mining Ophiomyia (Fig. 7), Melanagromyza (Fig. 2), Hexomyza or Phytomyza (Fig. 8). Wasps in the distinctive genus Trachionus (Fig. 9), are specialists on Phytobia. Some of the commonest of all parasitoids are relative polyphagous species of Dacnusa with a long pterostigma (Fig. 10) which can be found abundantly by sweeping low vegetation in summer.

Fig. 4. Chorebus lateralis (Braconidae, Alysiinae, Dacnusini), a common parasitoid of Agromyza spp. on nettles (Urtica). Chorebus is a huge genus with over 125 species in the British Isles alone. Most (but unfortunately not all) species have a metapleuron with a rosette of radially-arranged hairs (see A in inset) and a four-toothed mandible (see B).

Fig. 5. Dacnusa aquilegiae (male, left; female, right; Braconidae, Alysiinae, Dacnusini), a common parasitoid of Phytomyza aquilegiae. The best character separating Dacnusa from other genera (at least when specimens of both sexes are available) is that the venation differs between males and females with the male pterostigma (the dark cell on the leading edge of the forewing) darker and typically larger.

Fig. 6. Exotela spinifer (Braconidae, Alysiinae, Dacnusini), a parasitoid of Phytomyza cirsii, P. alpina, P. rydeniana & P. conyzae. The limits of the genus are hard to define though some species such as this one are easy to identify: unusually it has a spine sticking out dorsally from the thorax, visible in the photo behind the insertion of the hind wing, from which the species gets its name.

Fig. 7. Chorebus leptogaster (Braconidae, Alysiinae, Dacnusini), an Ophiomyia spp. parasitoid, a species with a characteristically long petiole – the stalk connecting the thorax and abdomen (or more accurately the mesasoma and metasoma).

Fig. 8. Chorebus crassipes (Braconidae, Alysiinae, Dacnusini), a parasitoid of Phytomyza diversicornis in stems of marsh lousewort (Pedicularis palustris); the long and robust ovipositor is unusual for the genus.

Fig. 9. Trachionus (=Symphya) hians (Braconidae, Alysiinae, Dacnusini) is one of three British species in the genus which together with the closely related Epimicta marginalis are parasitoids of Phytobia spp. feeding in woody cambium. Rearing records are scarce (this is a swept specimen) though S. hians has been reared from Phytobia cambii (=betulae) on birch. The wasps have distinctively sclerotised abdomens which may provide strengthened support for the ovipositor muscles to allow it to penetrate into wood.

Fig. 10. Female Dacnusa areolaris (Braconidae, Alysiinae, Dacnusini) which is an abundant parasitoid attacking the relatively polyphagous Chromatomyia syngenesiae and the grass specialist C. nigra (DNA sequence data supports the morphological conclusion that only one species is involved). The areolaris group of Dacnusa are characterized by a long pterostigma from which the radial vein originates from almost its base (see arrow). A closely related, and almost equally abundant species, D. maculipes, is unusual in being polyphagous and recorded from over 50 species of agromyzid.
The Dacnusini are members of the subfamily Alysiinae which have what are called exodont mandibles (Fig. 11) – flattened with three or four teeth. Typical braconid mandibles (Fig. 11) are more pointed at the end, not flattened. There are a few species of common agromyzid parasitoids (in the Dapsilarthra genus complex, Fig. 12) that are in the other Alysiinae tribe, the Alysiini. These have exodont mandible but possess the wing vein missing in the Dacnusini. The alysiine parasitoids attacking agromyzids have been monographed – superbly – by Graham Griffiths1-7.

Fig. 11. Mandibles of Alysiinae (left) and Opiinae (right), the two most frequent subfamilies of Braconidae attacking agromyzids. Alysiine have exodont mandibles which are flattened and swing widely out, and have 3 or 4 often co-equal teeth; they never touch or overlap when closed. Opiinae have endodont mandibles which are less flattened and meet when closed. The species on the left is Heterolexis balteata (see Fig. 12 legend) and that on the right Opius sp. nr. similis. The genus Opius in its modern sense is characterised by a flange on the ventral surface of the mandible indicated by an arrow.

Fig. 12. Heterolexis balteata (Braconidae, Alysiinae, Alysiini), a common parasitoid of larger Agromyzidae, chiefly Agromyza and Cerodontha spp.. The long antennae, yellow-marked abdomen, and the open subdiscal cell (see arrow) distinguish this species from other Alysiini attacking British agromyzids.
The second major group of braconids attacking agromyzids don’t have exodont mandibles and are in the subfamily Opiinae. Until recently, the 70 or so species in the UK were all placed in the genus Opius (Fig 3 & 11) but they are now distributed across 10 or so genera. Opiinae biology is less well known than Alysiinae but they seem to be a little less host specific. There is a marginally helpful European monograph8 but many UK opiines can only be identified by reference to the collections in the Dutch or Austrian national museums (I am slowly building a reference collection for the National Museum of Scotland).
There remains one other braconid that agromyzid workers will frequently encounter. This is the unusual Colastes braconius (Fig. 13) in the small subfamily Exothecinae. It is unusual because it is an ectoparasite and pupates in a silken cocoon in the mine, and because it is very polyphagous, even commoner on lepidopteran miners than dipteran. The elongate thorax and abdomen, with very few hairs, should distinguish this wasp from other braconids.

Fig. 13. Colastes braconius (Braconidae, Exotheciae), a polyphagous and common parasitoid of dipterous and lepidopterous leaf miners.
Chalcidoidea
The two major families attacking agromyzids are the Pteromalidae and Eulophidae. They are easy to tell apart if you have a binocular microscope: pteromalids have five tarsal segments and eulophids four (Fig.14). [If you have a series of obviously the same species in which the males have four segments, and females five, you haven’t gone mad but have Epiclerus panyas, the single Tetracampidae that attacks agromyzids.].
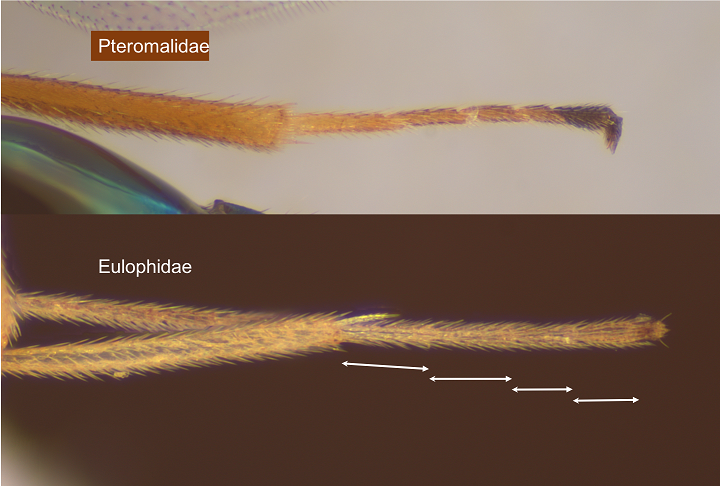
Fig. 14. Pteromalidae have five-segmented tarsi while Eulophidae have four segments. The pteromalid is Sphegigaster hexomyzae (see Fig. 18 legend) and the eulophid Chrysocharis orbicularis (see Fig. 2 legend)
There are also differences in antennal structure. Chalcidoid antennae typically have 7-13 segments. Starting from the head there is a long scape and then at a right angle a short pedicel; there are then a variable number of funicle segments followed by a three-segmented clava or club (the segments may be fused and difficult to distinguish). The pteromalids attacking agromyzids typically have 5-7 funicle segments (e.g. Fig. 18) and the eulophids 2-4 (e.g. Fig. 22-24).
Most pteromalids attacking leaf-mining agromyzids are in the subfamily Miscogasterinae characterised by asymmetric “teeth” on the lower margin of the face (clypeus) (Fig. 15). They are chiefly metallic green with complex sculpturing, some with noticeably stalked abdomens (long petioles) (Fig. 16). Stem-boring and galling agromyzids are also attacked by pteromalids from other subfamilies; they are and often very striking insects (Fig 17, 18). The males of some pteromalids (chiefly Halticoptera, Fig. 19) have very enlarged palps, looking like yellow bags under their head. British pteromalids are monographed by Graham9 with an easier key to genera by Boucek & Rasplus10.
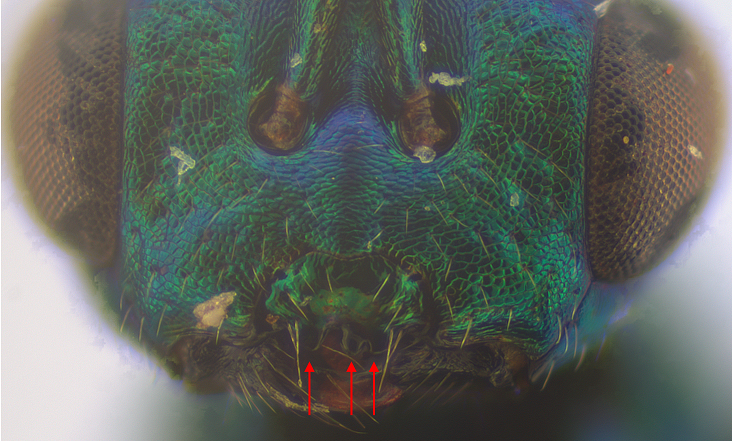
Fig. 15. Miscogasterinae (Pteromalidae) have asymmetric “teeth” on the ventral edge of their clypeus, typically one large one to the left (as you look at the face) and two smaller to the right (indicated by arrows). This is actually an anthomyiid parasitoid (Lamprotatus splendens) chosen to photograph as it is bigger than related agromyzid parasitoids.

Fig. 16. Miscogaster rufipes (Pteromalidae, Miscogasterinae), a relatively polyphagous endoparasitoid that is reared most frequently from Agromyza blotch mines. Miscogaster spp. are characterised by long petioles and wing with a large, globular stigma.

Fig. 17. Chlorocytus longicauda (Pteromalidae, Pteromalinae), a parasitoid of stem-mining Melanagromyza and Phytomyza spp.. The abdomen (metasoma) is particularly long in this species.

Fig. 18. This is a male Sphegigaster hexomyzae (Pteromalidae, Pteromalinae), a parasitoid of Hexomyza simplicoides on willows; it was described from Sweden in 1983 and found for the first time in the British Isles in 2018.

Fig. 19. Some male Halticoptera (Pteromalidae, Miscogasterinae) have enlarged yellow palps, as in this Halticoptera patellana reared from a Chirosia sp. (Anthomyiidae) on bracken (chosen as it illustrates the character better than in the agromyzid parasitoid specimens available).
The two main divisions of Eulophidae attacking agromyzids are Eulophinae Entedontinae. They can be told apart by the number of hairs on the dorsal surface of the submarginal vein: in the Entedontinae there are two (Fig. 20) while in the Eulophinae there are more and they are relatively shorter.

Fig. 20. Entedontinae have a pair of hairs on the dorsal surface of the marginal vein. The photograph is of Chrysocharis polyzo that chiefly attacks Cerodontha spp. on monocots.
Most Entedontinae attacking agromyzids are endoparasitoids in the large genus Chrysocharis (Fig. 2). These are small bluish green and green wasps, some with stalked and some with sessile abdomens. They tend not to be host specific but specialise on miners with similar biology, for example having blotch mines, or feeding on monocots. Other members of the genus attack leaf-mining moths. Pediobius metallicus is a more robust species that is a common polyphagous parasitoid of agromyzid (Fig. 21). There are keys to Entedontinae genera in Graham11 (and a Royal Entomological Society Handbook is in preparation), and Hansson12 monographs Chrysocharis.

Fig. 21. Pediobius mettalicus (=acantha) (Eulophidae, Entedontinae), a common parasitoid of a broad range of agromyzids.
In contrast to the Entedontinae, the Eulophinae are largely ectoparasites and pupate in the mine. Those that develop on early instar hosts can be very small. The commonest genera are Diglyphus (two funicle segments; Fig. 22), Hemiptarsenus (four funicle segments, scape long and protruding above top of head; Fig. 23) and Pnigalio (four funicle segments and short scape; Fig. 24). Hemiptarsenus females may be wingless, have banded wings, or have bright white terminal antennal segments (Fig. 25). Male Hemiptarsenus and Pnigalio have wonderful long branches (Fig. 26), presumably to increase their surface area to detect female pheromones. British Eulophinae are monographed by Askew13.

Fig. 22. Diglyphus isaea (Eulophidae, Eulophinae) (female), a very common polyphagous ectoparasitoid. The two-segmented funicle is characteristic of Diglyphus while the yellow venation and banding on the leg is unique to isaea. The wasp is reared commercially and sold as a biological control agent for greenhouse leaf miners. The heads of many small eulophids are thinly chitinised and tend to shrink after death, as can be seen in this and some of the other figures.

Fig. 23. Hemiptarsenus unguicellus (Eulophidae, Eulophinae) (female), a common ectoparasitoid of many agromyzids. Note the four-segmented funicle and long scape (first and longest antennal segment), characteristic of the genus.

Fig. 24. Pnigalio soemius (Eulophidae, Eulophinae) (female), a common polyphagous ectoparasitoid. Note the four-segmented funicle and, compared to Hemiptarsenus (Fig. 23), shorter scape.

Fig. 25. Hemiptarsenus fulvicollis (Eulophidae, Eulophinae) (female), a local ectoparasitoid attacking a broad range of agromyzids. This is a very characteristic species, extensively yellow with long antennae in which the final segment of the clava is white. Wasps ascribed to this species may be brachypterous (short-winged, as in this specimen) or fully winged though unpublished DNA sequence data suggest they may be different species.

Fig. 26. A male Pnigalio (Eulophidae, Eulophinae) showing the branched antennae found in a number of eulophid genera. Male Pnigalio can be difficult to identify but this specimen, reared from a lepidopterous leaf miner, is probably P. pectinicornis (this specimen shows its antennae better than specimens I have reared from agromyzids).
Recording
All records of parasitoids are valuable, especially where there is a firm host identification. Dipterists: please do not throw away parasitoids in disgust! Please send specimens to a hymenopterist you know or to me. Vouchers of those sent to me* will end up in the collection of the National Museum of Scotland at Edinburgh where Mark Shaw has built up a major collection of reared UK parasitoids. I try to get identifications back to people who send specimens reasonably quickly.
The easiest way temporarily to preserve parasitoids for posting is dry in vials between loosely packed tissue paper. Never use cotton wool as the fibres get caught in the specimen. Handle parasitoids by picking them up by the wing using watchmakers’ forceps or equivalents; avoid picking them up by the antennae or legs. Most of the parasitoids described here can only be identified when dry so storing in alcohol is not advised unless for a specific purpose such as DNA genome analysis.
Naturally, provide full data for the specimen. It cannot be stressed too strongly that accurate data is to be preferred. Thus “Agromyzidae ex Holcus mollis” or “Most likely Liriomyza flaveola ex Holcus sp.” is much more valuable than an unconfirmed but definite “Liriomyza flaveola ex Holcus mollis”. A high proportion of records in the literature are inaccurate (records of parasitoids on leaf-miner websites have not been critically appraised and must be used with caution).
Agromyzid parasitoids are permanently preserved mounted on cards or points. The insect is glued on its side such that characters on all other aspects can be seen clearly. Mounting these insects well takes some practice and it is very easy to obscure important characters, especially around the mouth, with glue. If you are not confident then store unmounted.
* Post to Charles Godfray, Oxford Martin School, Oxford University, 34 Broad St, Oxford OX1 3BD.
Parasitoid wish list
Below is a list of agromyzids from which reared parasitoids are particularly interesting. The list is biased towards Dacnusini (Braconidae; parasitoids mentioned below belong to this taxon unless otherwise indicated) because I am trying to build a DNA sequence library of European species but also include Opiinae (Braconidae), Miscogasterinae and Pteromalinae (both Pteromalidae). Though there is much remaining to be discovered, parasitoids of agromyzids are relatively well-known, as are the larger but related parasitoids of leaf-mining Anthomyiidae and Tephritidae. Related parasitoids attacking leaf-mining Hydrellia (Ephydridae) on aquatic plants and stem-boring Chloropidae are more much poorly known and rearings from these hosts would be very interesting.
General
Parasitoids from rare or unusual hosts are obviously interesting. More generally, there are relatively few records from non-leaf-mining agromyzids in Napomyza, Phytobia, Hexomyza, Ophiomyia and Melanagromyza, galling Agromyza and seed-head Phytomyza. Among leaf-miners, we know least about those mining sedges and grasses and the parasitoids of Cerodontha are particularly interesting.
Specific
Agromyza abiens on Boraginaceae. The common braconid parasitoid of this host is Dacnusa abdita but D. maxima has also been reared from it. The two are probably conspecific, with maxima as the name suggests a large variant, and DNA sequence from fresh specimens of maxima may help resolve this.
Agromyza albitarsis on Salix repens. Fresh specimens of its poorly-known parasitoid Chorebus perkinsi for DNA sequencing.
Agromyza nigrescens on Geranium spp. A Phaedrotoma (Opiinae) from this host might be P. pulchriceps but fresh specimens for DNA sequencing would be helpful.
Agromyza pseudorufipes on Myosotis scorpiodes. One specimen of an undescribed Eurytenes (nr. macrocerus, Opiinae) has been reared from this host.
Agromyza spp. leaf-mining shrubby Fabaceae (e.g. A. johannae & A. pulla). Fresh specimens needed of their tiny parasitoid Chorebus lar which has proved hard so far to extract DNA from. Further specimens of a Stictomischus sp. (Miscogasterinae) which has been reared from A. johannae would be useful.
Agromyza spp. mining grass. A number of Chorebus spp. and Protodacnusa spp. attack these agromyzids but are relatively poorly known and lack DNA sequences.
Agromyza viciae & vicifoliae on Vicia. Two poorly known Exotela (dives and viciae) have been reared from these miners and further specimens for morphological and DNA study are needed. More specimens of an Opiostomus, possibly impatientis, and an Apodesmia (nr. curtipectus) (both Opiinae), from this host would be valuable.
Aulagromyza heringii on Fraxinus. Further specimens of an Opiostomus (Opiinae) reared from this host would be helpful.
Cerodontha (Poemyza) superciliosa leaf-mining Ammophila arenaria on sand dunes. Fresh specimens of its parasitoid Chorebus vitripennis for DNA sequencing.
Cerodontha lateralis. A single Chorebus fordii has been reared from this host on Agrostis but DNA sequencing failed and further fresh specimens are needed.
Cerodontha on Cyperaceae & Juncaceae. Resolution of several taxonomic issues would be helped by reared new specimens, especially if host is definitely known.
Cerodontha subgenus Cerodontha. There are a group of small elongate Chorebus that are swept quite commonly and which are probably all parasitoids of these common but rarely-reared sheath-mining agromyzids; rearing records and DNA sequencing would be very helpful for resolving several taxonomic issues.
Liriomyza, Aulagromyza and Ophiomyia spp. attacking Galium. Parasitoids of these hosts very poorly known.
Hexomyza sarothamni on Cytisus. One literature parasitoid record from over 100 years ago that can be linked to a modern species; any rearings will be fascinating.
Hexomyza schineri on Populus. Fresh specimens of its parasitoid Chorebus gedanensis for DNA sequencing. Sphegigaster glabrata parasitises this host as does possible S. truncata which is on the British list but has not been reared in this country.
Liriomyza artemisicola and probably other Liriomyza on Artemisia. Fresh specimens of the parasitoid Chorebus artemisiellus for DNA sequencing.
Liriomyza cicerina on Ononis spp.. Commonly attacked by an undescribed Chorebus (near pseudomisellus), further specimens of which would be helpful. Further specimens of an Opius sp. (Opiinae) reared from this host would be interesting.
Liriomyza mesnili on liverworts. This species, the only agromyzid to attack liverworts, is not recorded from the British Isles. However, Coloneura stylata, which parasitises this host on the continent has. Coloneura are tiny and rather poorly known parasitoids and stylata may have a different host here (or two species may be involved) but worth scanning liverworts.
Liriomyza pascuum on Euphorbia. I suspect Chorebus incertus from this host is conspecific with C. daimenes but have failed so far to extract DNA and would welcome fresh specimens.
Liriomyza polygalae on Polygala. Two (damaged) specimens of a very small Dacnusa that may be undescribed have been reared from this host and further specimens would be very interesting.
Liriomyza spp. leaf-mining Hieracium. Fresh specimens of the poorly-known parasitoids Chorebus cambricus & C. thecla for DNA sequencing.
Liriomyza spp. mining Triglochin. A single damaged Opius from this host isn’t immediately ascribable to known species. No other parasitoids known from these saltmarsh species.
Liriomyza valerianella on Valerianella sp.. An undescribed Chorebus (near daimenes) has been reared from this host in the UK which DNA sequence data suggest is quite common in continental Europe; further specimens would be helpful.
Liriomyza virgo in stems of Equisetum fluviatile. Fresh specimens of the parasitoid Dacnusa obesa for DNA sequencing. Any other parasitoids from Equisetum miners would be interesting.
Metopomyza flavonotata leaf-mining Poaceae. Fresh specimens of its poorly-known parasitoid Chorebus varuna for DNA sequencing.
Napomyza cichorii. Fresh specimens of its common parasitoid Chorebus glaber to get DNA sequence to compare with rearings of this species from other hosts.
Ophiomyia heracleivora on Heracleum. Fresh specimens of its parasitoid Chorebus heracleivora for DNA sequencing.
Ophiomyia orbiculata on Pisum. Fresh specimens of its parasitoid Chorebus orbiculatae for DNA sequencing (known only from this host but doesn’t seem very common).
Hexomyza (= Ophiomyia) simplex on Asparagus. Fresh specimens of its common parasitoid Chorebus rondanii to get DNA sequence to compare with swept material.
Ophiomyia spp. in Leontodon, Hypochoeris & Picris midribs. Two males have been reared in the UK of a Chorebus near senilis from Leontodon that may be undescribed but females needed to pursue further. Chorebus pulchellus is known from Germany from an unknown agromyzid attacking Hypochoeris that may be an Ophiomyia, and C. xiphidius in Germany from an Ophiomyia on Picris; confirmations of host and DNA sequences needed.
Ophiomyia spp. in Sonchus midribs. The common parasitoid is Chorebus leptogaster but a second Chorebus has been reared that is probably undescribed though seems to be widespread (there are specimens with the same DNA barcode from Turkey, Russia and Canada). It may be more common on other less frequently reared Ophiomyia spp.
Phytomyza aconiti on Delphinium and Aconitum. Fresh specimens of its parasitoid Dacnusa delphinii for DNA sequencing.
Phytomyza affinis in seedheads of Euphrasia spp.. Fresh specimens of its parasitoids Dacnusa euphasiella & nigropygmaea for DNA sequencing. A single specimen of what I think is Opius breviscapus (Opiinae) has been reared from this host but further specimens are needed to confirm (would be new to British list).
Phytomyza albiceps [=rydeniana]) on Asteraceae. Attacked by an undescribed Dacnusa (near alpestris), further specimens of which would be helpful. More specimens of a single Opiostomus sp. (Opiinae) from this host would also be interesting.
Phytomyza buhriella (=notabalis) in stems of Petasites albus (Asteraceae). Fresh specimens of its parasitoid Dacnusa merope (reared from this host in Germany) for DNA sequencing.
Phytomyza calthivora leaf-mining Caltha palustris. Fresh specimens of its parasitoid Chorebus calthae for DNA sequencing.
Phytomyza cecidonomia on Asteraceae. Discrepancies between morphological and DNA sequencing data of its parasitoid Chorebus merion may be resolved by new reared specimens, especially if they are female. DNA sequencing suggests Dacnusa pubescens from this host is distinct from specimens reared from Napomyza and further specimens would be helpful.
Phytomyza cirsii on Cirsium spp.. Attacked by an undescribed Chorebus (near alecto), further specimens of which would be helpful.
Phytomyza diversicornis in stems of Pedicularis palustris. Attacked by an undescribed Dacnusa (near centaureae), further specimens of which would be helpful. There is a species of the difficult genus Seladerma (Miscogasterinae) on this host, further specimens of which would also be helpful.
Phytomyza lithospermi [not a British species] leaf-mining Lithospermum officinale. Chorebus thecla has been reared from this host in Germany but has a curiously disjoint host distribution which DNA sequencing may help explain.
Phytomyza pedicularifolia on Pedicularis spp.. Attacked by a probably undescribed Chorebus (near mitra), further specimens of which would be helpful.
Phytomyza pimpinellae leaf-mining Pimpinella spp. I suspect its parasitoid Chorebus pimpinellae may be a variant of C. armida which attacks related hosts on umbels and DNA sequences from fresh specimens may help resolve this.
Phytomyza plantaginis on Plantago maritima. More specimens of a Halticoptera sp. (Miscogasterinae) reared from this host would be interesting.
Phytomyza rufipes in stems and midribs of Brassica. Fresh specimens of its parasitoid Chorebus thusa for DNA sequencing.
Phytomyza scabiosae on Scabiosa columbaria. Fresh specimens of its parasitoid Chorebus scabiosae for DNA sequencing.
Phytomyza silai on Silaum. Coloneura dice has been reared from this host, a member of a genus with no DNA sequence so far.
Phytomyza soenderupi from stems of Caltha. There is a species of the difficult genus Seladerma (Miscogasterinae) attacking this host, further specimens of which would be helpful. Opius soenderupianus (Opiinae) was described from this host but is on the British list from specimens reared from Phytomyza orobanchia; I suspect two species are involved. Finally it would be good to confirm a Stenomalina (?gracilis) (Pteromalinae) from this host.
Phytomyza tenella in seed heads of Pedicularis. Both Dacnusa monticola & nigella are recorded from this host but there are other agromyzids in this niche. Parasitoids, ideally, with their host puparium, needed for DNA sequencing.
Phytomyza thysselini on Peucedanum. A single Exotela that may be cyclogaster has been reared from this host but further specimens (and DNA sequencing which failed on the lone specimen) are needed to resolve.
Phytomyza varipes in Rhinanthus spp. seed capsules. A single specimen of what may be Opius caudifer (Opiinae) has been reared from this host and further specimens would be helpful.
References
1 Griffiths, G. C. D. The Alysiinae (Hym. Braconidae) parasites of the Agromyzidae (Diptera). VII. Supplement. Beiträge zur Entomologie 34, 343-362 (1984).
2 Griffiths, G. C. D. The Alysiinae (Hym. Braconidae) parasites of the Agromyzidae (Diptera). VI. The parasites of Cerodontha Rondani. Beiträge zur Entomologie 18, 63-152 (1968).
3 Griffiths, G. C. D. The Aiysiinae (Hym. Braconidae) parasites of the Agromyzidae (Diptera). V. The parasites of Liriomyza Mik and certain small genera of Phytomyzinae. Beiträge zur Entomologie 18, 5-62 (1968).
4 Griffiths, G. C. D. The Alysiinae (Hym. Braconidae) parasites of the Agromyzidae (Diptera). IV. The parasites of Hexomyza Enderlein, Melanagromyza Hendel, Ophiomyia Braschnikov and Napomyza Westwood. Beiträge zur Entomologie 17, 653-696 (1967).
5 Griffiths, G. C. D. The Alysiinae (Hym. Braconidae) parasites of the Agromyzidae (Diptera). II. The parasites of Agromyza Fallén. Beiträge zur Entomologie 16, 551-605 (1966).
6 Griffiths, G. C. D. The Alysiinae (Hym. Braconidae) parasites of the Agromyzidae (Diptera). I. General questions of taxonomy, biology and evolution. Beiträge zur Entomologie 14, 823-914 (1964).
7 Griffiths, G. C. D. The Alysiinae (Hym. Braconidae) parasites of the Agromyzidae (Diptera). III. The parasites of Paraphytomyza Enderlein, Phytagromyza Hendel and Phytomyza Fallén. Beiträge zur Entomologie 16, 775-951 (1966).
8 Fischer, M. Hymenoptera, Braconidae (Opiinae I). Das Tierreich 91, 1-620 (1972).
9 Graham, M. W. R. D. V. The Pteromalidae of Northwestern Europe (Hymenoptera: Chalcidoidea). Bulletin of the British Museum (Natual History) Supplement 16, 1-908 (1969).
10 Bouček, Z. & Rasplus, J.-Y. Illustrated Key to West-Palaearctic Genera of Pteromalidae (Hymenoptera: Chalcidoidea). (Institut National de la Recherche Agronomique, 1991).
11 Graham, M. W. R. D. Keys to the British genera and species of Elachertinae, Eulophinae, Entedontinae and Euderinae (Hym., Chalcidoidea). Transactions of the Society for British Entomology 13, 169-204 (1959).
12 Hansson, C. Taxonomy and biology of the Palearctic species of Chrysocharis Foerster, 1856 (Hymenoptera: Eulophidae). Entomologica Scandinavica Suppl. 26, 1-130 (1985).
13 Askew, R. R. Elasmidae and Eulophidae (Elachertinae, Eulophinae, Euderinae). Handbooks for the Identification of British Insects VIII 2(b). (Royal Entomological Society, 1969).


